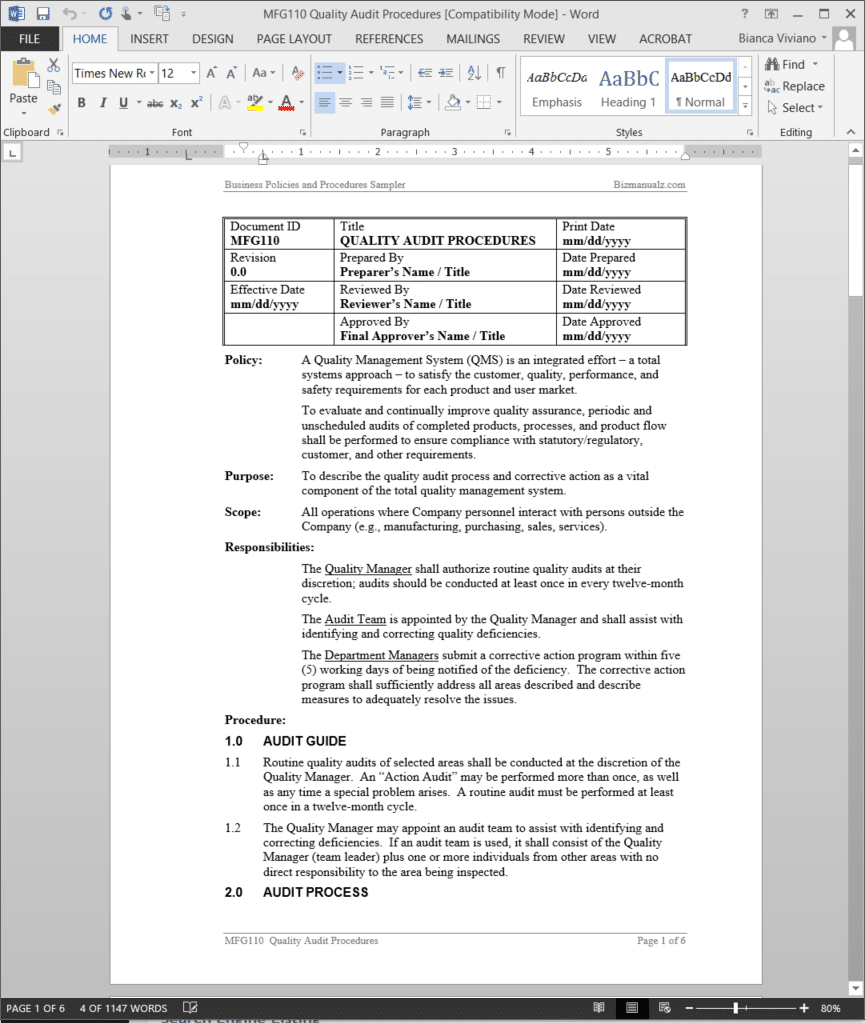
Quality Audit Procedure | MFG110
 Quality Audit Procedure
Quality Audit Procedure
The Quality Audit Procedure can be used to describe the quality audit process and corrective action as a vital component of the total quality management system.
A Quality Management System (QMS) is an integrated effort – a total systems approach – to satisfy the customer, quality, performance, and safety requirements for each product and user market. To evaluate and continually improve quality assurance, periodic and unscheduled audits of completed products, processes, and product flow shall be performed to ensure compliance with statutory/regulatory, customer, and other requirements.
The Quality Audit Procedure applies to all operations where company personnel interact with persons outside the company (e.g., manufacturing, purchasing, sales, services). (6 pages, 1147 words)
Quality Audit Responsibilities:
The Quality Manager shall authorize routine quality audits at their discretion; audits should be conducted at least once in every twelve-month cycle.
The Audit Team is appointed by the Quality Manager and shall assist with identifying and correcting quality deficiencies.
The Department Managers submit a corrective action program within five (5) working days of being notified of the deficiency. The corrective action program shall sufficiently address all areas described and describe measures to adequately resolve the issues.
 Quality Audit Procedure Activities
Quality Audit Procedure Activities
- Audit Guide
- Audit Process
- Corrective Action
- Audit Records
Quality Audit Procedure Forms






