Water quality standards: Biochemical oxygen demand – Responsible Seafood Advocate
Mục lục
Through its Responsible Aquaculture Program, GAA has recommended a range of water quality standards for shrimp farm effluents. Part of a series, this article addresses Biochemical oxygen demand.
Biochemical oxygen demand (BOD) is an index of the oxygen demand in wastewater. Although it is an important water quality variable used in effluent management and regulation, most aquaculturists have little experience with BOD.
Measuring BOD
BOD is measured by confining a sample of water in a bottle in the dark at 20 degrees-C for a specific period of time, and determining the amount of dissolved oxygen consumed by microorganisms in the water. Freshwater contains 9.08 milligrams per liter dissolved oxygen at saturation, while under the same conditions, seawater will contain 7.38 milligrams per liter. The oxygen demand of water confined in a BOD bottle must not exceed the saturation concentration for dissolved oxygen at 20 degrees-C, or the water will become depleted of dissolved oxygen and BOD measurement will be impossible.
Sample preparation
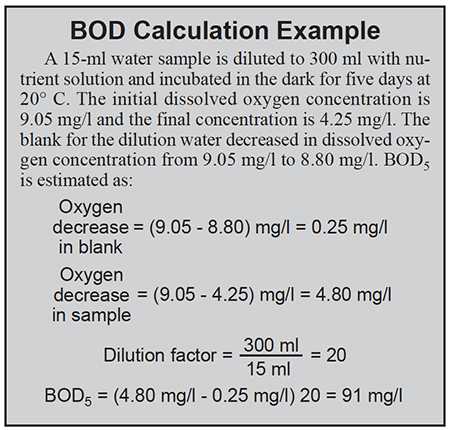
Samples expected to have a high BOD must be diluted. When samples must be diluted more than two or three times, the concentration of nutrients and bacteria will be diluted greatly. Nutrients and bacterial seed must be added to dilution water to prevent dilution effects from lessening the rate of expression of BOD. A blank to measure oxygen demand of dilution water is necessary.
Test duration
The usual length of BOD analysis is five days, with the abbreviation BOD5 used to denote this practice. Ultimate BOD (BODu) is the total oxygen demand of a sample. Its value is several times the five-day BOD.
Water must be incubated 30 days or more in the ultimate BOD test. To prevent oxygen depletion, the water must be aerated one or more times. Thus, the ultimate BOD is time-consuming and difficult to measure. It is not used nearly as often as BOD5 testing.
Processes that control BOD
The BOD of a water sample results from oxidation of soluble and particulate organic matter to carbon dioxide and water by bacteria, oxidation of ammonia nitrogen to nitrate by nitrifying organisms, and respiration by plankton organisms in samples.
Nitrification and BOD
Nitrification can begin immediately in samples with little dilution. Where samples are diluted many times in the BOD analysis, it will take five days or more for populations of nitrifying organisms to develop and begin to oxidize ammonia.
Sometimes a nitrification inhibitor is added to BOD samples to stop oxygen consumption by nitrifying bacteria. When this practice is followed, the BOD is called the carbonaceous BOD or CBOD. Nitrification can be a significant contributor to BOD, for oxidation of 1 milligrams per liter of ammonia nitrogen requires 4.58 milligrams per liter dissolved oxygen.
Aquaculture pond samples
Samples from aquaculture ponds usually do not need to be diluted more than several fold, and nitrification begins immediately in the BOD test. Nitrification often represents 30 to 40 percent of BOD5 in pond water samples, and the use of nitrification inhibitor is not recommended.
Effluent BOD and oxygen demand
For effluent with greater BOD values, larger amounts of dissolved oxygen will be required to oxidize the organic matter and ammonia nitrogen that results from the release of the effluent into natural waters. Of course, the oxygen demand will depend both on the concentration of five-day BOD and the volume of effluent (BOD5 in milligrams per liter x effluent volume in cubic meters per day = BOD5 load in grams per day).
Load limits
 In some cases, it is possible to estimate how much BOD can be added to a water body without depressing dissolved-oxygen concentrations to harmful levels. Thus, water quality permits sometimes specify a maximum daily BOD load. In cases where the assimilative capacity of the receiving water is large or effluent volume is small, a very large BOD can be permitted without exceeding the load limit.
In some cases, it is possible to estimate how much BOD can be added to a water body without depressing dissolved-oxygen concentrations to harmful levels. Thus, water quality permits sometimes specify a maximum daily BOD load. In cases where the assimilative capacity of the receiving water is large or effluent volume is small, a very large BOD can be permitted without exceeding the load limit.
A large BOD can result in dissolved-oxygen depletion in the mixing zone where effluent is diluted by the receiving water. For this reason, permits may specify both a maximum daily load and a maximum BOD concentration. The usual five-day BOD limit for effluents is 20 or 30 milligrams per liter, but concentrations up to 50 milligrams per liter are sometimes allowed.
BOD in aquaculture ponds
In aquaculture ponds, BOD5 rarely exceeds 30 milligrams per liter, and most values will be below 10 milligrams per liter. The oxygen demand is expressed more slowly in aquaculture pond effluents than in most wastewater, because the main source of oxygen demand is respiration by living plankton in pond effluents. Thus, dissolved-oxygen depletion in the mixing zone is not a major concern.
The Global Aquaculture Alliance adopted an initial BOD5 limit of 50 milligrams per liter, with a target limit of 30 milligrams per liter. Even in intensive shrimp culture, the target BOD concentration should be achievable.
Practices to reduce BOD

Suggested practices to reduce biochemical oxygen demand in ponds include:
- Do not stock and feed at rates that cause serious water quality deterioration.
- Use high-quality feed, and apply good feed
management to prevent overfeeding.
- Do not use fertilizers, except as necessary
to maintain phytoplankton blooms.
- Use adequate mechanical aeration in intensive farms.
- Where possible, discharge water through
sedimentation basins.
- Allow a fallow period between crops, and lime acidic bottom soil to encourage decomposition of soil
organic matter between crops.
Compliance with GAA standards
To comply with GAA standards, conventional five-day BOD without nitrification inhibition should be used. The amount of dilution will vary, but effluent-to-dilution water ratios of 1:1, 1:2, 1:4 and 1:8 are suggested. After the technician gains experience in the relationship between sample appearance and BOD concentration, fewer dilutions will be necessary.

As far as equipment, a 20 degrees-C incubator is essential, and it is highly desirable to use a polargraphic dissolved-oxygen meter with BOD bottle probe. Hach Chemical Co. of Loveland, Colorado, USA and other suppliers sell equipment and supplies for BOD analysis. Standard Methods for the Examination of Water and Wastewater and other water-analysis manuals provide further details for BOD analysis.
(Editor’s Note: This article was originally published in the October 2001 print edition of the Global Aquaculture Advocate.)
Now that you’ve reached the end of the article …
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Support GSA and Become a Member
Related Posts

Responsibility
Accuracy of custom water analyses varies
The reliability of trace element analyses reported by custom laboratories cannot be checked by simple techniques, and results may not always be accurate. One should check the reliability of major ion analyses by determining the charge balance and comparing the measured total ion concentration with the total ion concentration estimated from conductivity.

Responsibility
Calcium and magnesium use in aquaculture
Aquatic plants and animals get the essential nutrients calcium and magnesium from water and food. Calcium concentrations impact the hydration and development of eggs in a hatchery, where calcium carbonate precipitation can be troublesome.

Responsibility
Effluent effects from aquaculture ponds
In general, lower-intensity pond and cage farming tends to discharge higher overall pollution loads in farm effluents than closed aquaculture systems.

Responsibility
Water quality standards: pH
The pH of shrimp pond water is influenced by source water, pH and acidity of bottom soil, shrimp culture inputs and biological activity.






