Controlling the 5Ms in Pursuit of Product Quality | Part 5 – Method – Opcenter
There are some basic tenets of manufacturing that we’ve been reviewing in this blog series. Control of the 5Ms of manufacturing – material, man, machine, measure, and method – is critical for creating quality products. Build it right the first time, every time. The difference in getting back to basics today is that manufacturers have access to technology to automate those controls, namely a Manufacturing Execution System.
In the final part of this series, we focus on Method, the defined procedures for building the product. The goal of method control is to prevent an operator from making a mistake in the method in which he or she assembles the product. Those procedures are typically documented in work instructions, or the SOP.
While the MES can link to a document management system to display the latest approved procedures, it can also enforce the use of proper procedures. With electronic procedures functionality, the MES can break the procedure down into structured step-by-step instructions, rather than an unstructured “paper on glass” method of displaying word or pdf documents.
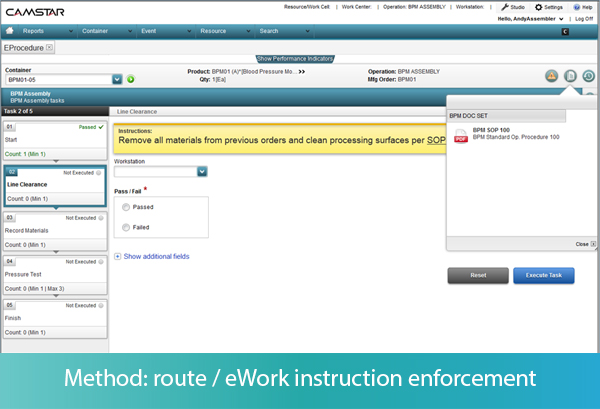 In an “eProcedure,” each step of the method can have attributes associated with it, such as whether the step is required or optional, whether the step can be performed once or several times, or whether the steps must be performed in sequential order or can be performed in random order. As each step on the MES screen is performed, the operator inputs the successful completion of the step. The data about the process is captured, and the MES validates whether that data is within spec.
In an “eProcedure,” each step of the method can have attributes associated with it, such as whether the step is required or optional, whether the step can be performed once or several times, or whether the steps must be performed in sequential order or can be performed in random order. As each step on the MES screen is performed, the operator inputs the successful completion of the step. The data about the process is captured, and the MES validates whether that data is within spec.
Let’s take some examples. As each step of a procedure is presented to the operator, he or she must input that the task has been started. If the task is not an approved next step as defined by the work instructions or SOP, the MES generates an error message to the operator before the operator can perform the task, preventing deviations in method. Every action, along with identification of the operator and the timestamp, is recorded in the MES.
The MES can also take the operator down different workflows, depending on the outcome of a specific step. For example, if the task is to visually inspect a component, the operator must input Pass or Fail to complete the task. Pass will take him to the next step, while Fail may take him to a nonconformance screen. The operator may also be instructed to input multiple measurements during an activity, which the MES may use to calculate a result, such as the average of the width measure of 2 samples. If the calculation is out of spec, the MES may trigger a nonconformance record, or move the item to a rework location, depending on the rules defined in the system
You can see why an MES is the heart of quality manufacturing – it is the system that controls the 5Ms in pursuit of product quality. At the same time, it records and tracks all data, automatically generating a Device History Record or Batch Record. The MES not only ensures compliance with regulation, but gets to the intent of ensuring product quality. And that’s basic manufacturing excellence.
Read More about Camstar MES for Medical devices
Get Started
What to read next
Benefits of the Digital Thread in CPG Manufacturing
Siemens Digital Industries Software helps CPG manufacturers react quickly to demanding market requirements with a new level of flexibility, scale…

Digital Manufacturing for the Automotive Industry
Using a comprehensive portfolio of integrated solutions to accelerate innovation and drive quality Highly disruptive trends in the automotive industry…

The critical role of quality control and inspection planning
Quality control and inspection planning plays a critical role in quality programs, not only to ensure that you produce high-quality…






